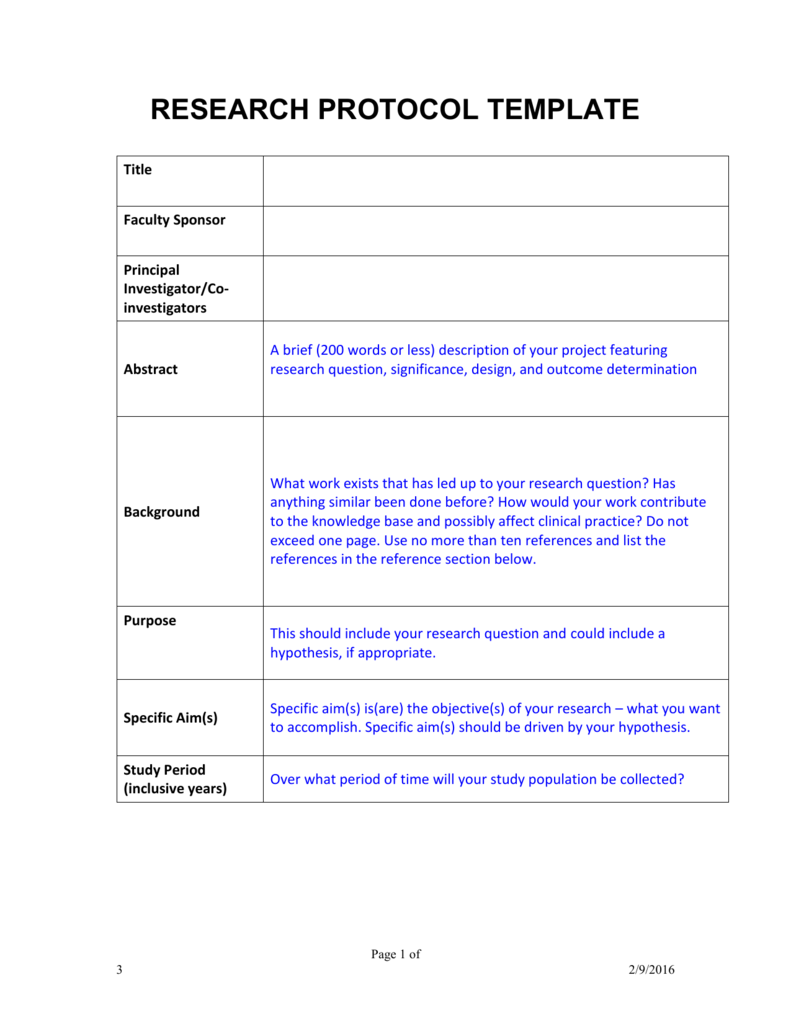
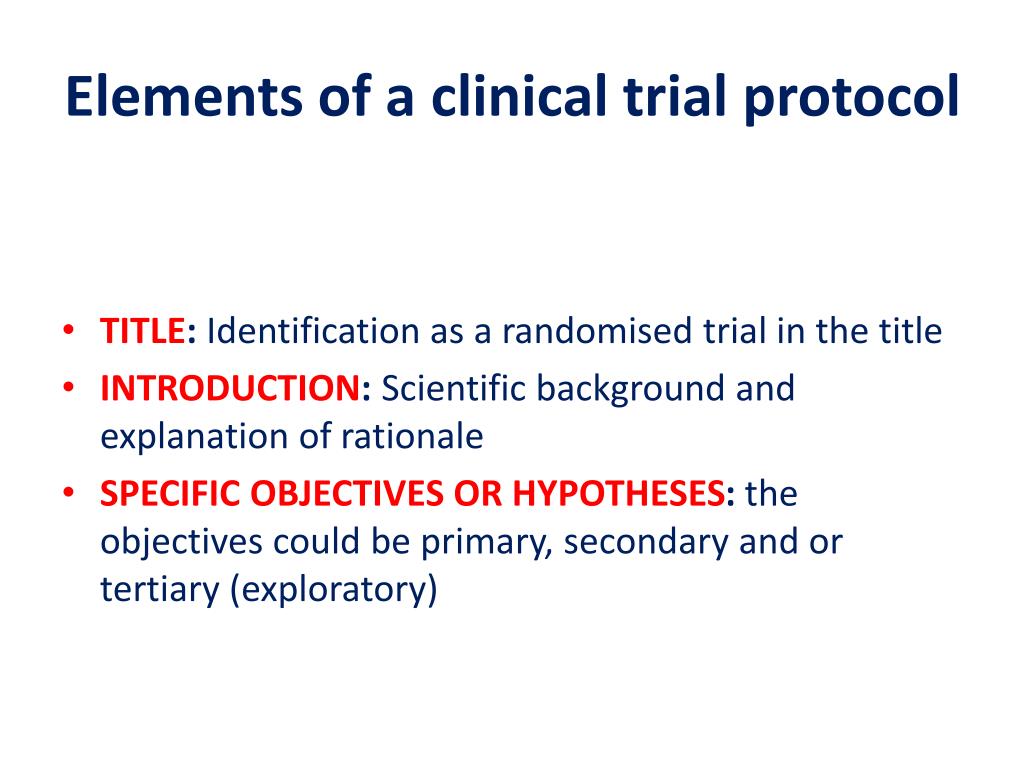
Clinical Research Protocol Template - Nih applicants can use a template with instructional and sample text to help write clinical protocols for the following types of research:. Clinical trial protocol template this protocol template is designed to help research teams develop a clinical trial protocol that includes an. Please note that this page has been updated for 2015 following a quality check. The natural history/observational protocol template, the. Protocol a written account of all the procedures to be followed in a trial, which describes all the administrative, documentation, analytical and. They follow the format of typical nih and industry multicenter protocols. The irb provides several protocol templates on this page. Nimh encourages investigators to consider using one of the protocol templates below when developing a clinical research protocol. There are three templates to be used for observational research: Welcome to global health trials' tools and templates library.
They follow the format of typical nih and industry multicenter protocols. Please note that this page has been updated for 2015 following a quality check. There are three templates to be used for observational research: The irb provides several protocol templates on this page. Welcome to global health trials' tools and templates library. The natural history/observational protocol template, the. Nimh encourages investigators to consider using one of the protocol templates below when developing a clinical research protocol. Nih applicants can use a template with instructional and sample text to help write clinical protocols for the following types of research:. Protocol a written account of all the procedures to be followed in a trial, which describes all the administrative, documentation, analytical and. Clinical trial protocol template this protocol template is designed to help research teams develop a clinical trial protocol that includes an.
Nimh encourages investigators to consider using one of the protocol templates below when developing a clinical research protocol. The natural history/observational protocol template, the. They follow the format of typical nih and industry multicenter protocols. There are three templates to be used for observational research: Clinical trial protocol template this protocol template is designed to help research teams develop a clinical trial protocol that includes an. Welcome to global health trials' tools and templates library. Nih applicants can use a template with instructional and sample text to help write clinical protocols for the following types of research:. Protocol a written account of all the procedures to be followed in a trial, which describes all the administrative, documentation, analytical and. Please note that this page has been updated for 2015 following a quality check. The irb provides several protocol templates on this page.
research protocol template
They follow the format of typical nih and industry multicenter protocols. Protocol a written account of all the procedures to be followed in a trial, which describes all the administrative, documentation, analytical and. There are three templates to be used for observational research: Nih applicants can use a template with instructional and sample text to help write clinical protocols for.
Research Protocol Template Guide for Researchers
Nih applicants can use a template with instructional and sample text to help write clinical protocols for the following types of research:. They follow the format of typical nih and industry multicenter protocols. The natural history/observational protocol template, the. Nimh encourages investigators to consider using one of the protocol templates below when developing a clinical research protocol. The irb provides.
PPT Elements of a clinical trial research protocol PowerPoint
The irb provides several protocol templates on this page. There are three templates to be used for observational research: The natural history/observational protocol template, the. They follow the format of typical nih and industry multicenter protocols. Nih applicants can use a template with instructional and sample text to help write clinical protocols for the following types of research:.
Template Device protocol
They follow the format of typical nih and industry multicenter protocols. There are three templates to be used for observational research: Welcome to global health trials' tools and templates library. Nih applicants can use a template with instructional and sample text to help write clinical protocols for the following types of research:. Clinical trial protocol template this protocol template is.
WA Health Research Protocol Template for Clinical Trials
Please note that this page has been updated for 2015 following a quality check. The irb provides several protocol templates on this page. Nimh encourages investigators to consider using one of the protocol templates below when developing a clinical research protocol. They follow the format of typical nih and industry multicenter protocols. The natural history/observational protocol template, the.
Clinical trial protocol vector infographic template 2308775 Vector Art
Welcome to global health trials' tools and templates library. The irb provides several protocol templates on this page. There are three templates to be used for observational research: They follow the format of typical nih and industry multicenter protocols. The natural history/observational protocol template, the.
Clinical Trial Protocol Template Word
Clinical trial protocol template this protocol template is designed to help research teams develop a clinical trial protocol that includes an. The irb provides several protocol templates on this page. Nimh encourages investigators to consider using one of the protocol templates below when developing a clinical research protocol. Protocol a written account of all the procedures to be followed in.
Phase 1 Clinical Trial Protocol Template
Clinical trial protocol template this protocol template is designed to help research teams develop a clinical trial protocol that includes an. Protocol a written account of all the procedures to be followed in a trial, which describes all the administrative, documentation, analytical and. Nimh encourages investigators to consider using one of the protocol templates below when developing a clinical research.
instructions for clinical research protocol template Doc Template
The irb provides several protocol templates on this page. Please note that this page has been updated for 2015 following a quality check. Welcome to global health trials' tools and templates library. Nih applicants can use a template with instructional and sample text to help write clinical protocols for the following types of research:. Clinical trial protocol template this protocol.
Clinical Trial Protocol Template Word
Clinical trial protocol template this protocol template is designed to help research teams develop a clinical trial protocol that includes an. Please note that this page has been updated for 2015 following a quality check. Nih applicants can use a template with instructional and sample text to help write clinical protocols for the following types of research:. The natural history/observational.
They Follow The Format Of Typical Nih And Industry Multicenter Protocols.
The natural history/observational protocol template, the. Please note that this page has been updated for 2015 following a quality check. Nimh encourages investigators to consider using one of the protocol templates below when developing a clinical research protocol. Welcome to global health trials' tools and templates library.
Clinical Trial Protocol Template This Protocol Template Is Designed To Help Research Teams Develop A Clinical Trial Protocol That Includes An.
Nih applicants can use a template with instructional and sample text to help write clinical protocols for the following types of research:. There are three templates to be used for observational research: Protocol a written account of all the procedures to be followed in a trial, which describes all the administrative, documentation, analytical and. The irb provides several protocol templates on this page.