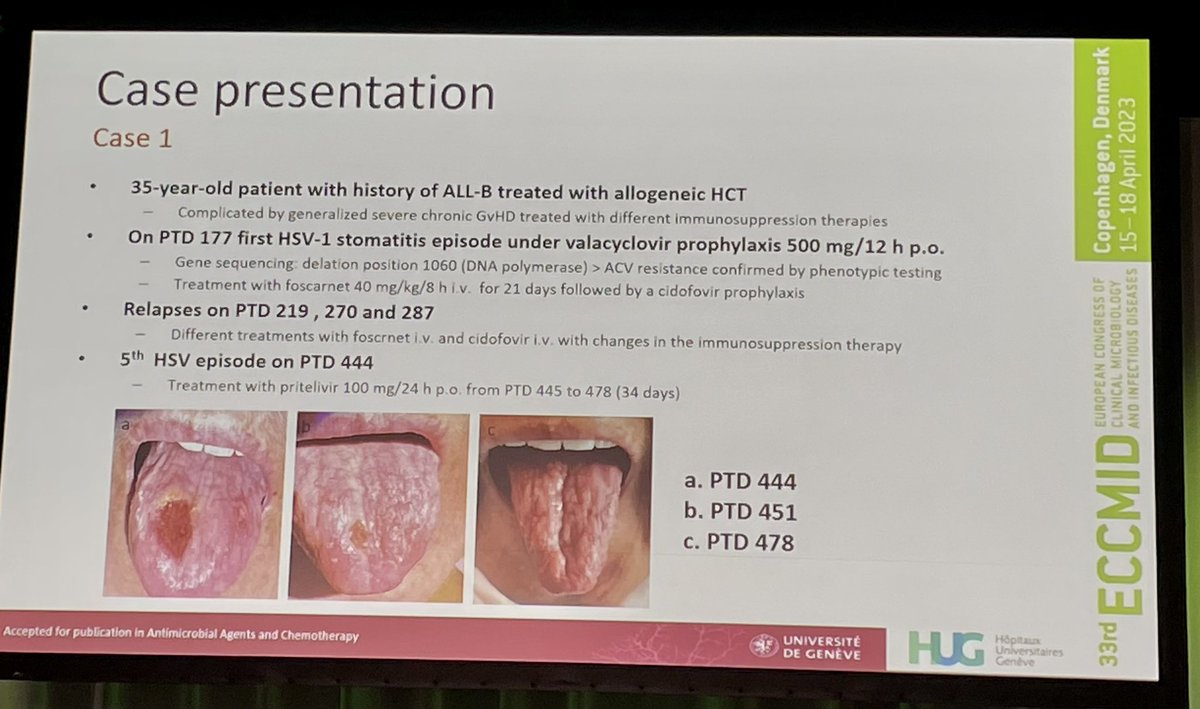
Is Pritelivir Available In Usa - The timeline is contingent upon the successful. Oral pritelivir is currently in a phase 2 clinical trial in the u.s., to assess efficacy and safety comparing pritelivir to i.v. By granting expanded access, accelerated approval, and priority review for pritelivir, the fda has the opportunity to alleviate the profound. Eligible patients for an eap are in high medical need, cannot participate in a clinical trial, and have exhausted all registered treatment options. Pritelivir is anticipated to be available by late 2023 or early 2024, subject to regulatory approvals.
The timeline is contingent upon the successful. By granting expanded access, accelerated approval, and priority review for pritelivir, the fda has the opportunity to alleviate the profound. Pritelivir is anticipated to be available by late 2023 or early 2024, subject to regulatory approvals. Eligible patients for an eap are in high medical need, cannot participate in a clinical trial, and have exhausted all registered treatment options. Oral pritelivir is currently in a phase 2 clinical trial in the u.s., to assess efficacy and safety comparing pritelivir to i.v.
Eligible patients for an eap are in high medical need, cannot participate in a clinical trial, and have exhausted all registered treatment options. Pritelivir is anticipated to be available by late 2023 or early 2024, subject to regulatory approvals. The timeline is contingent upon the successful. Oral pritelivir is currently in a phase 2 clinical trial in the u.s., to assess efficacy and safety comparing pritelivir to i.v. By granting expanded access, accelerated approval, and priority review for pritelivir, the fda has the opportunity to alleviate the profound.
Pritelivir, Thermo Scientific™ Fisher Scientific
The timeline is contingent upon the successful. Oral pritelivir is currently in a phase 2 clinical trial in the u.s., to assess efficacy and safety comparing pritelivir to i.v. Pritelivir is anticipated to be available by late 2023 or early 2024, subject to regulatory approvals. By granting expanded access, accelerated approval, and priority review for pritelivir, the fda has the.
Pritelivir for Herpes Simplex Clinical Trial 2022 Power
Eligible patients for an eap are in high medical need, cannot participate in a clinical trial, and have exhausted all registered treatment options. The timeline is contingent upon the successful. By granting expanded access, accelerated approval, and priority review for pritelivir, the fda has the opportunity to alleviate the profound. Pritelivir is anticipated to be available by late 2023 or.
pritelivir (AIC316) / AiCuris
Eligible patients for an eap are in high medical need, cannot participate in a clinical trial, and have exhausted all registered treatment options. Pritelivir is anticipated to be available by late 2023 or early 2024, subject to regulatory approvals. By granting expanded access, accelerated approval, and priority review for pritelivir, the fda has the opportunity to alleviate the profound. Oral.
pritelivir (AIC316) / AiCuris
Oral pritelivir is currently in a phase 2 clinical trial in the u.s., to assess efficacy and safety comparing pritelivir to i.v. The timeline is contingent upon the successful. By granting expanded access, accelerated approval, and priority review for pritelivir, the fda has the opportunity to alleviate the profound. Eligible patients for an eap are in high medical need, cannot.
pritelivir Semantic Scholar
Pritelivir is anticipated to be available by late 2023 or early 2024, subject to regulatory approvals. By granting expanded access, accelerated approval, and priority review for pritelivir, the fda has the opportunity to alleviate the profound. Eligible patients for an eap are in high medical need, cannot participate in a clinical trial, and have exhausted all registered treatment options. Oral.
Pritelivir Application in Therapy and Current Clinical Research
Eligible patients for an eap are in high medical need, cannot participate in a clinical trial, and have exhausted all registered treatment options. Pritelivir is anticipated to be available by late 2023 or early 2024, subject to regulatory approvals. By granting expanded access, accelerated approval, and priority review for pritelivir, the fda has the opportunity to alleviate the profound. The.
Mathematical modeling of herpes simplex virus2 suppression with
Pritelivir is anticipated to be available by late 2023 or early 2024, subject to regulatory approvals. Oral pritelivir is currently in a phase 2 clinical trial in the u.s., to assess efficacy and safety comparing pritelivir to i.v. The timeline is contingent upon the successful. By granting expanded access, accelerated approval, and priority review for pritelivir, the fda has the.
HelicasePrimase Inhibitor Pritelivir for HSV2 Infection New England
The timeline is contingent upon the successful. Eligible patients for an eap are in high medical need, cannot participate in a clinical trial, and have exhausted all registered treatment options. By granting expanded access, accelerated approval, and priority review for pritelivir, the fda has the opportunity to alleviate the profound. Pritelivir is anticipated to be available by late 2023 or.
Discovery, Chemistry, and Preclinical Development of Pritelivir, a
The timeline is contingent upon the successful. Pritelivir is anticipated to be available by late 2023 or early 2024, subject to regulatory approvals. Oral pritelivir is currently in a phase 2 clinical trial in the u.s., to assess efficacy and safety comparing pritelivir to i.v. Eligible patients for an eap are in high medical need, cannot participate in a clinical.
Plasma concentrationtime curves for pritelivir after oral
Oral pritelivir is currently in a phase 2 clinical trial in the u.s., to assess efficacy and safety comparing pritelivir to i.v. By granting expanded access, accelerated approval, and priority review for pritelivir, the fda has the opportunity to alleviate the profound. Pritelivir is anticipated to be available by late 2023 or early 2024, subject to regulatory approvals. The timeline.
The Timeline Is Contingent Upon The Successful.
By granting expanded access, accelerated approval, and priority review for pritelivir, the fda has the opportunity to alleviate the profound. Eligible patients for an eap are in high medical need, cannot participate in a clinical trial, and have exhausted all registered treatment options. Pritelivir is anticipated to be available by late 2023 or early 2024, subject to regulatory approvals. Oral pritelivir is currently in a phase 2 clinical trial in the u.s., to assess efficacy and safety comparing pritelivir to i.v.