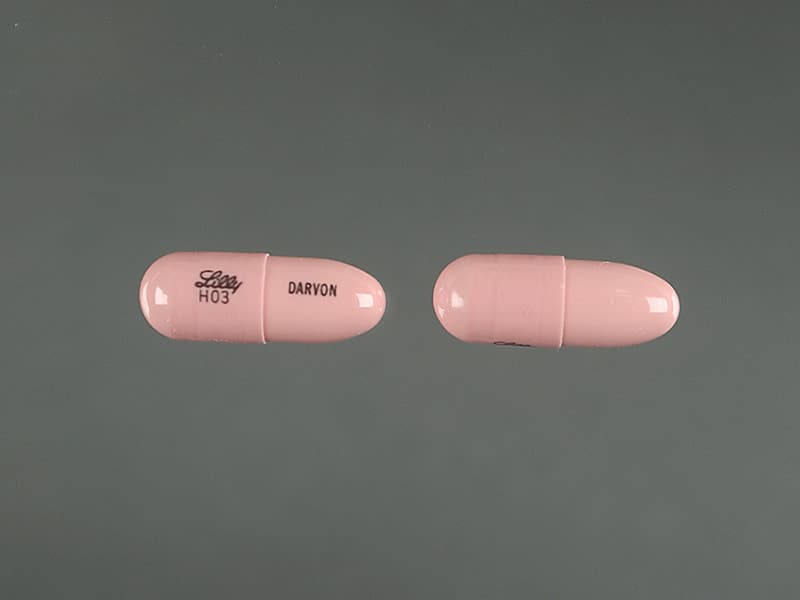
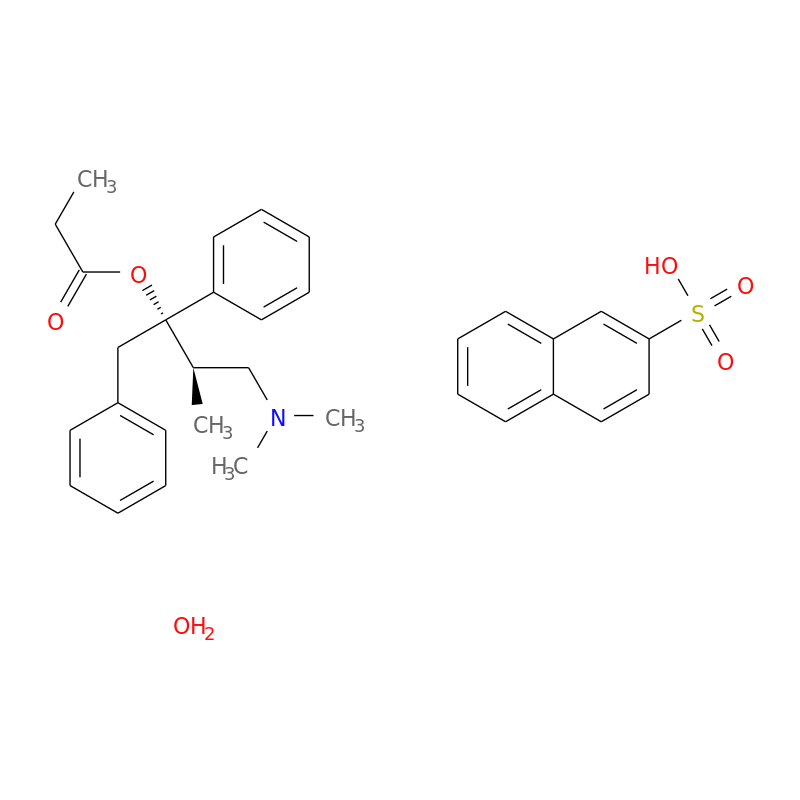
Is Propoxyphene Still Available - The agency has also asked makers of generic drugs. The fda has announced the withdrawal of all propoxyphene products from the us market (darvon, darvocet) due to risk of arrythmias. It was that study that led to the drug being pulled from the market friday.
It was that study that led to the drug being pulled from the market friday. The fda has announced the withdrawal of all propoxyphene products from the us market (darvon, darvocet) due to risk of arrythmias. The agency has also asked makers of generic drugs.
It was that study that led to the drug being pulled from the market friday. The fda has announced the withdrawal of all propoxyphene products from the us market (darvon, darvocet) due to risk of arrythmias. The agency has also asked makers of generic drugs.
Propoxyphene Drug Test Included in a 10 Panel Drug Test
The fda has announced the withdrawal of all propoxyphene products from the us market (darvon, darvocet) due to risk of arrythmias. It was that study that led to the drug being pulled from the market friday. The agency has also asked makers of generic drugs.
PROPOXYPHENE Generic Name, Drug class, Brande Name ,Precautions
The agency has also asked makers of generic drugs. It was that study that led to the drug being pulled from the market friday. The fda has announced the withdrawal of all propoxyphene products from the us market (darvon, darvocet) due to risk of arrythmias.
PPT A Comprehensive Review of Treating Acute Pain PowerPoint
It was that study that led to the drug being pulled from the market friday. The agency has also asked makers of generic drugs. The fda has announced the withdrawal of all propoxyphene products from the us market (darvon, darvocet) due to risk of arrythmias.
OPIATES Mrs.Farina. ppt download
The fda has announced the withdrawal of all propoxyphene products from the us market (darvon, darvocet) due to risk of arrythmias. It was that study that led to the drug being pulled from the market friday. The agency has also asked makers of generic drugs.
Propoxyphene brand name list from
The fda has announced the withdrawal of all propoxyphene products from the us market (darvon, darvocet) due to risk of arrythmias. The agency has also asked makers of generic drugs. It was that study that led to the drug being pulled from the market friday.
OPIATES Mrs.Farina. ppt download
The agency has also asked makers of generic drugs. It was that study that led to the drug being pulled from the market friday. The fda has announced the withdrawal of all propoxyphene products from the us market (darvon, darvocet) due to risk of arrythmias.
What is Propoxyphene YouTube
It was that study that led to the drug being pulled from the market friday. The agency has also asked makers of generic drugs. The fda has announced the withdrawal of all propoxyphene products from the us market (darvon, darvocet) due to risk of arrythmias.
PPT Pain Management Updates and Issues PowerPoint Presentation, free
The fda has announced the withdrawal of all propoxyphene products from the us market (darvon, darvocet) due to risk of arrythmias. The agency has also asked makers of generic drugs. It was that study that led to the drug being pulled from the market friday.
DailyMed PROPOXYPHENE propoxyphene hydrochloride capsule
The agency has also asked makers of generic drugs. The fda has announced the withdrawal of all propoxyphene products from the us market (darvon, darvocet) due to risk of arrythmias. It was that study that led to the drug being pulled from the market friday.
It Was That Study That Led To The Drug Being Pulled From The Market Friday.
The fda has announced the withdrawal of all propoxyphene products from the us market (darvon, darvocet) due to risk of arrythmias. The agency has also asked makers of generic drugs.