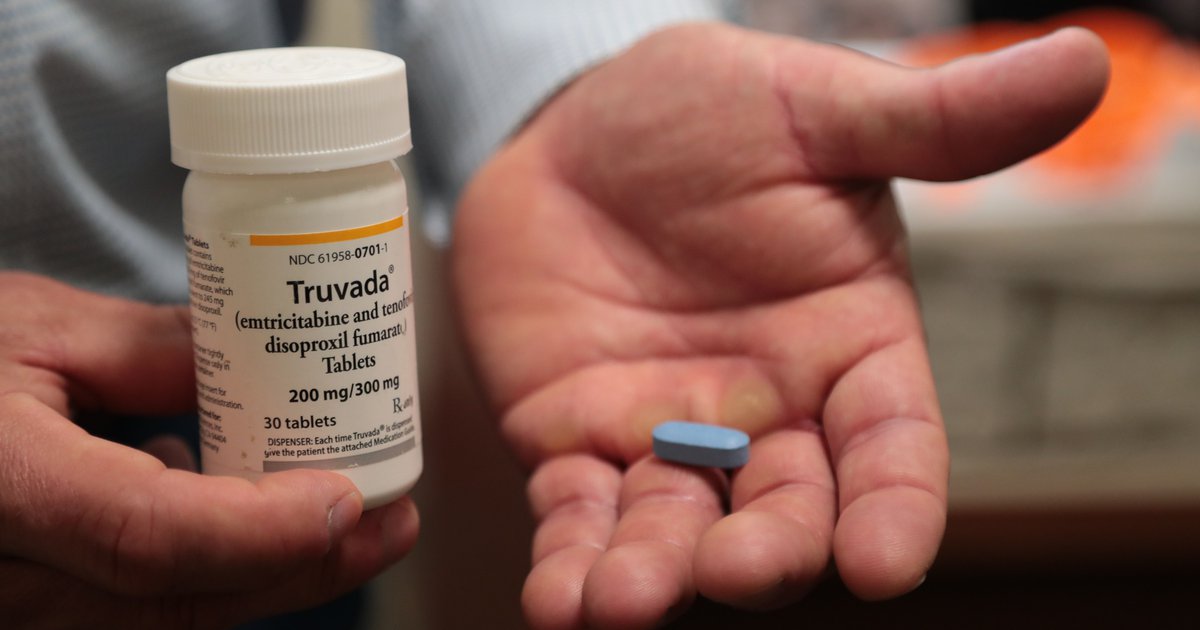
When Did Prep Become Available - Although hiv prep medications are only available in oral tablet and injectable formulations, other formulations are being developed and studied. In 2019, descovy was added as a prep option, and the. In july 2012, the united states (u.s.) food and drug administration (fda) approved the first medication for use as hiv pre. Truvada was the first medication approved for hiv prevention 11 years ago. In 2012, significantly reducing hiv transmission risk.
In 2012, significantly reducing hiv transmission risk. Although hiv prep medications are only available in oral tablet and injectable formulations, other formulations are being developed and studied. In july 2012, the united states (u.s.) food and drug administration (fda) approved the first medication for use as hiv pre. In 2019, descovy was added as a prep option, and the. Truvada was the first medication approved for hiv prevention 11 years ago.
In 2019, descovy was added as a prep option, and the. In 2012, significantly reducing hiv transmission risk. In july 2012, the united states (u.s.) food and drug administration (fda) approved the first medication for use as hiv pre. Although hiv prep medications are only available in oral tablet and injectable formulations, other formulations are being developed and studied. Truvada was the first medication approved for hiv prevention 11 years ago.
FDA approves Apretude, first injectable PrEP treatment to lower risk of
In july 2012, the united states (u.s.) food and drug administration (fda) approved the first medication for use as hiv pre. In 2012, significantly reducing hiv transmission risk. In 2019, descovy was added as a prep option, and the. Truvada was the first medication approved for hiv prevention 11 years ago. Although hiv prep medications are only available in oral.
A “Prevention Revolution” Offers Hope in the World’s Largest HIV
Although hiv prep medications are only available in oral tablet and injectable formulations, other formulations are being developed and studied. In 2012, significantly reducing hiv transmission risk. In july 2012, the united states (u.s.) food and drug administration (fda) approved the first medication for use as hiv pre. Truvada was the first medication approved for hiv prevention 11 years ago..
Preexposure prophylaxis (PrEP) AIDS Network
In july 2012, the united states (u.s.) food and drug administration (fda) approved the first medication for use as hiv pre. Although hiv prep medications are only available in oral tablet and injectable formulations, other formulations are being developed and studied. Truvada was the first medication approved for hiv prevention 11 years ago. In 2019, descovy was added as a.
Preexposure prophylaxis (PrEP) AIDS Network
Truvada was the first medication approved for hiv prevention 11 years ago. In july 2012, the united states (u.s.) food and drug administration (fda) approved the first medication for use as hiv pre. Although hiv prep medications are only available in oral tablet and injectable formulations, other formulations are being developed and studied. In 2012, significantly reducing hiv transmission risk..
When Was Prep Invented
In 2019, descovy was added as a prep option, and the. Truvada was the first medication approved for hiv prevention 11 years ago. Although hiv prep medications are only available in oral tablet and injectable formulations, other formulations are being developed and studied. In 2012, significantly reducing hiv transmission risk. In july 2012, the united states (u.s.) food and drug.
PrEP in Wales marking one year since the HIV prevention pill became
In july 2012, the united states (u.s.) food and drug administration (fda) approved the first medication for use as hiv pre. In 2019, descovy was added as a prep option, and the. Truvada was the first medication approved for hiv prevention 11 years ago. In 2012, significantly reducing hiv transmission risk. Although hiv prep medications are only available in oral.
PrEP Facts What are the ways to take PrEP? San Francisco AIDS Foundation
Truvada was the first medication approved for hiv prevention 11 years ago. Although hiv prep medications are only available in oral tablet and injectable formulations, other formulations are being developed and studied. In july 2012, the united states (u.s.) food and drug administration (fda) approved the first medication for use as hiv pre. In 2012, significantly reducing hiv transmission risk..
PrEP vs. PEP Infographic How It Works, Who It's For, and More Prep
In july 2012, the united states (u.s.) food and drug administration (fda) approved the first medication for use as hiv pre. In 2012, significantly reducing hiv transmission risk. Truvada was the first medication approved for hiv prevention 11 years ago. Although hiv prep medications are only available in oral tablet and injectable formulations, other formulations are being developed and studied..
What Will My Child Learn in Prep? And how you can help.
In 2019, descovy was added as a prep option, and the. Truvada was the first medication approved for hiv prevention 11 years ago. In july 2012, the united states (u.s.) food and drug administration (fda) approved the first medication for use as hiv pre. In 2012, significantly reducing hiv transmission risk. Although hiv prep medications are only available in oral.
Why I PrEP AID Atlanta
Although hiv prep medications are only available in oral tablet and injectable formulations, other formulations are being developed and studied. In 2019, descovy was added as a prep option, and the. In 2012, significantly reducing hiv transmission risk. In july 2012, the united states (u.s.) food and drug administration (fda) approved the first medication for use as hiv pre. Truvada.
In 2019, Descovy Was Added As A Prep Option, And The.
In july 2012, the united states (u.s.) food and drug administration (fda) approved the first medication for use as hiv pre. In 2012, significantly reducing hiv transmission risk. Truvada was the first medication approved for hiv prevention 11 years ago. Although hiv prep medications are only available in oral tablet and injectable formulations, other formulations are being developed and studied.