When Will Saracatinib Be Available For Ipf - When i read about sarcatinib, i found that the trial is call, stop ipf. Recent investigations from tulane university reveal that saracatinib, initially developed for oncology, may offer a new. The us food and drug administration (fda) has granted orphan drug designation (odd) for saracatinib, a potential. In the trial, which began in 2020, ipf patients receive either saracatinib or a placebo. However, i cannot see any preliminary reports, and may. The stop ipf trial is expected to be completed within a year from now, which suggests that results may be available by late 2025 [7]. The trial’s primary outcome is safety;.
The stop ipf trial is expected to be completed within a year from now, which suggests that results may be available by late 2025 [7]. The us food and drug administration (fda) has granted orphan drug designation (odd) for saracatinib, a potential. In the trial, which began in 2020, ipf patients receive either saracatinib or a placebo. Recent investigations from tulane university reveal that saracatinib, initially developed for oncology, may offer a new. When i read about sarcatinib, i found that the trial is call, stop ipf. However, i cannot see any preliminary reports, and may. The trial’s primary outcome is safety;.
However, i cannot see any preliminary reports, and may. The stop ipf trial is expected to be completed within a year from now, which suggests that results may be available by late 2025 [7]. Recent investigations from tulane university reveal that saracatinib, initially developed for oncology, may offer a new. In the trial, which began in 2020, ipf patients receive either saracatinib or a placebo. When i read about sarcatinib, i found that the trial is call, stop ipf. The us food and drug administration (fda) has granted orphan drug designation (odd) for saracatinib, a potential. The trial’s primary outcome is safety;.
A schematic model of the antifibrotic mechanism of Saracatinib
Recent investigations from tulane university reveal that saracatinib, initially developed for oncology, may offer a new. The stop ipf trial is expected to be completed within a year from now, which suggests that results may be available by late 2025 [7]. The trial’s primary outcome is safety;. In the trial, which began in 2020, ipf patients receive either saracatinib or.
Saracatinib suppressed Fyn/FAK/NWASP pathway. Western blot analysis on
The trial’s primary outcome is safety;. The stop ipf trial is expected to be completed within a year from now, which suggests that results may be available by late 2025 [7]. However, i cannot see any preliminary reports, and may. When i read about sarcatinib, i found that the trial is call, stop ipf. Recent investigations from tulane university reveal.
A schematic model of the antifibrotic mechanism of Saracatinib
When i read about sarcatinib, i found that the trial is call, stop ipf. In the trial, which began in 2020, ipf patients receive either saracatinib or a placebo. The stop ipf trial is expected to be completed within a year from now, which suggests that results may be available by late 2025 [7]. However, i cannot see any preliminary.
Saracatinib is a selective inhibitor of BMP versus TGFβ type I
However, i cannot see any preliminary reports, and may. When i read about sarcatinib, i found that the trial is call, stop ipf. Recent investigations from tulane university reveal that saracatinib, initially developed for oncology, may offer a new. The trial’s primary outcome is safety;. The us food and drug administration (fda) has granted orphan drug designation (odd) for saracatinib,.
Antioxidants Free FullText Saracatinib, a Src Tyrosine Kinase
The us food and drug administration (fda) has granted orphan drug designation (odd) for saracatinib, a potential. The trial’s primary outcome is safety;. The stop ipf trial is expected to be completed within a year from now, which suggests that results may be available by late 2025 [7]. In the trial, which began in 2020, ipf patients receive either saracatinib.
Saracatinib for Idiopathic Pulmonary Fibrosis Clinical Trial 2025 Power
When i read about sarcatinib, i found that the trial is call, stop ipf. In the trial, which began in 2020, ipf patients receive either saracatinib or a placebo. However, i cannot see any preliminary reports, and may. The us food and drug administration (fda) has granted orphan drug designation (odd) for saracatinib, a potential. Recent investigations from tulane university.
HNSCC cells exhibit differential response to saracatinib. a The effect
In the trial, which began in 2020, ipf patients receive either saracatinib or a placebo. The trial’s primary outcome is safety;. However, i cannot see any preliminary reports, and may. Recent investigations from tulane university reveal that saracatinib, initially developed for oncology, may offer a new. When i read about sarcatinib, i found that the trial is call, stop ipf.
SRCinhibitor saracatinib abrogates bronchosphere formation af
In the trial, which began in 2020, ipf patients receive either saracatinib or a placebo. Recent investigations from tulane university reveal that saracatinib, initially developed for oncology, may offer a new. When i read about sarcatinib, i found that the trial is call, stop ipf. The trial’s primary outcome is safety;. The stop ipf trial is expected to be completed.
Saracatinib enhances the efficacy of ispinesib in murine and human GBM
However, i cannot see any preliminary reports, and may. In the trial, which began in 2020, ipf patients receive either saracatinib or a placebo. Recent investigations from tulane university reveal that saracatinib, initially developed for oncology, may offer a new. The us food and drug administration (fda) has granted orphan drug designation (odd) for saracatinib, a potential. The trial’s primary.
SRCinhibitor saracatinib attenuates fibrosis in the humanized mouse
However, i cannot see any preliminary reports, and may. The trial’s primary outcome is safety;. In the trial, which began in 2020, ipf patients receive either saracatinib or a placebo. The us food and drug administration (fda) has granted orphan drug designation (odd) for saracatinib, a potential. Recent investigations from tulane university reveal that saracatinib, initially developed for oncology, may.
When I Read About Sarcatinib, I Found That The Trial Is Call, Stop Ipf.
The us food and drug administration (fda) has granted orphan drug designation (odd) for saracatinib, a potential. Recent investigations from tulane university reveal that saracatinib, initially developed for oncology, may offer a new. In the trial, which began in 2020, ipf patients receive either saracatinib or a placebo. The trial’s primary outcome is safety;.
The Stop Ipf Trial Is Expected To Be Completed Within A Year From Now, Which Suggests That Results May Be Available By Late 2025 [7].
However, i cannot see any preliminary reports, and may.




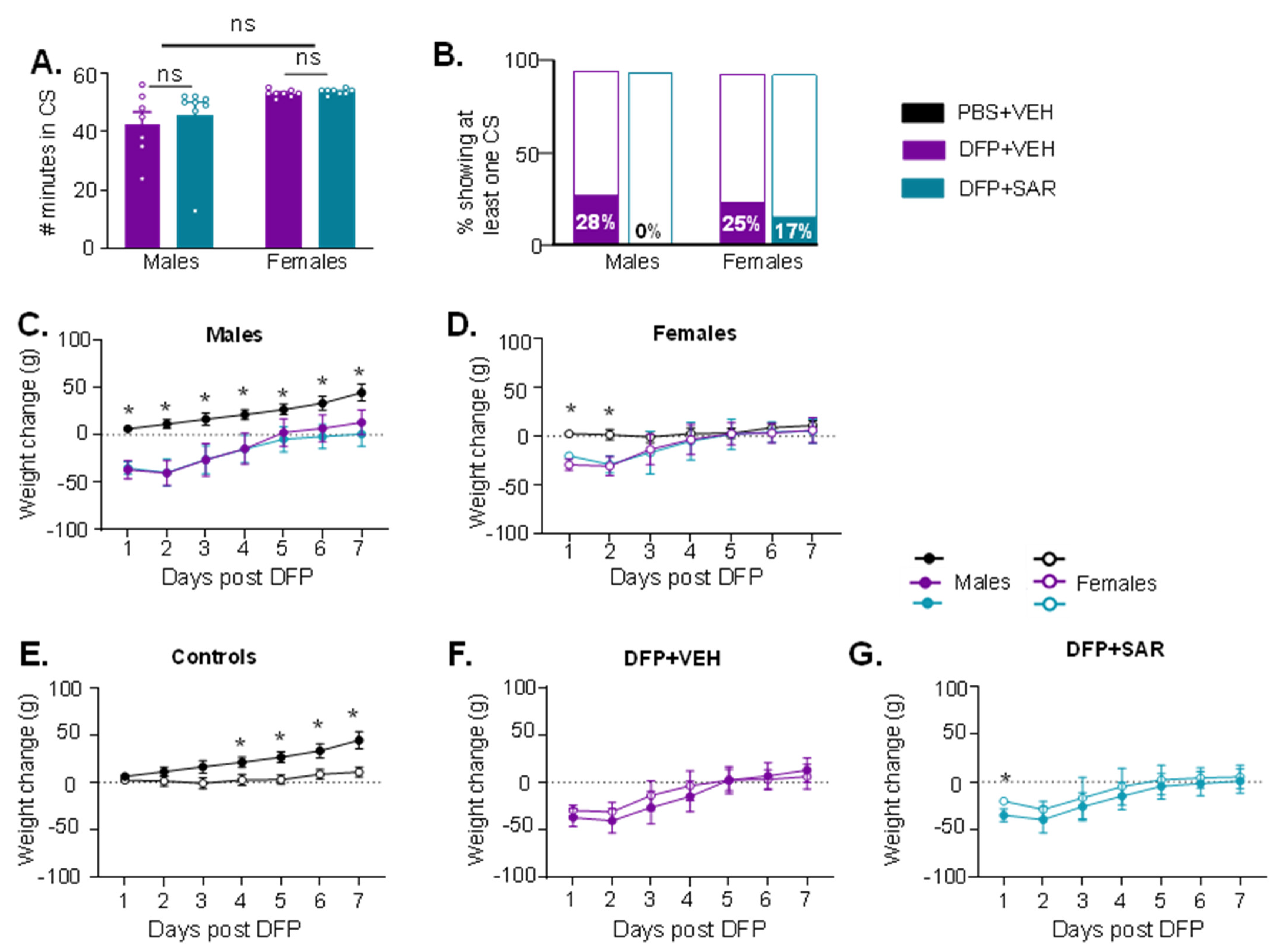
:%0A%0ASaracatinib for Idiopathic Pulmonary Fibrosis.png?md=1)



