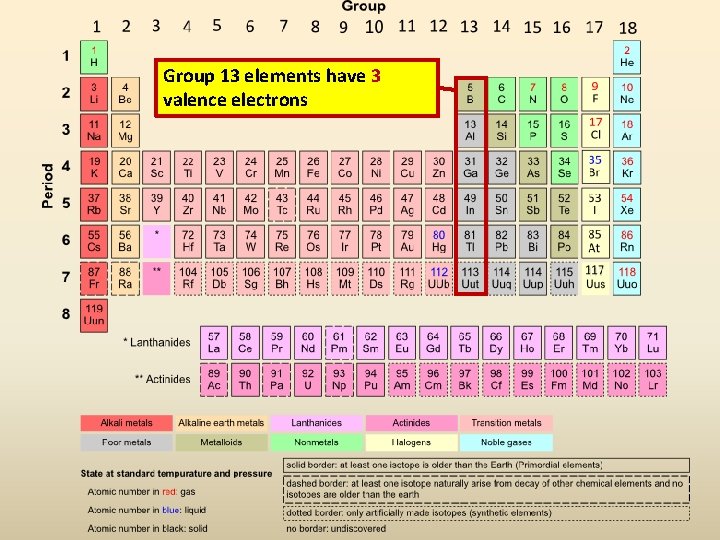
Which Element Has The Fewest Valence Electrons Available For Bonding - This is typical for elements in group. The element with the fewest valence electrons available for bonding is lithium (li), located in group 1, which has 1 valence. The total number of electrons present in the outer. A) iodine b) aluminum c) carbon d) nitrogen Which element has the fewest valence electrons available for bonding? Carbon (c) has 4 valence electrons. Thus, aluminum (al) has the fewest valence electrons, with only 3 available for bonding. Aluminum (al) has 3 valence electrons. Sodium (na) with atomic number 11 has the least number of valence electrons. Nitrogen (n) has 5 valence electrons.
Aluminum (al) has 3 valence electrons. A) iodine b) aluminum c) carbon d) nitrogen Study with quizlet and memorize flashcards containing terms like how many valence electrons are available for bonding in bromine (br)?,. The element with the fewest valence electrons available for bonding is lithium (li), located in group 1, which has 1 valence. Carbon (c) has 4 valence electrons. This is typical for elements in group. Which element has the fewest valence electrons available for bonding? Sodium (na) with atomic number 11 has the least number of valence electrons. The total number of electrons present in the outer. Nitrogen (n) has 5 valence electrons.
Study with quizlet and memorize flashcards containing terms like how many valence electrons are available for bonding in bromine (br)?,. Aluminum (al) has 3 valence electrons. Sodium (na) with atomic number 11 has the least number of valence electrons. The total number of electrons present in the outer. Thus, aluminum (al) has the fewest valence electrons, with only 3 available for bonding. A) iodine b) aluminum c) carbon d) nitrogen This is typical for elements in group. Which element has the fewest valence electrons available for bonding? Carbon (c) has 4 valence electrons. The element with the fewest valence electrons available for bonding is lithium (li), located in group 1, which has 1 valence.
Elements and Chemical Bonds
Iodine (i) has 7 valence electrons. Which element has the fewest valence electrons available for bonding? Aluminum (al) has 3 valence electrons. The total number of electrons present in the outer. Carbon (c) has 4 valence electrons.
PPT Bonding PowerPoint Presentation, free download ID6281432
Study with quizlet and memorize flashcards containing terms like how many valence electrons are available for bonding in bromine (br)?,. This is typical for elements in group. Nitrogen (n) has 5 valence electrons. Aluminum (al) has 3 valence electrons. Iodine (i) has 7 valence electrons.
Lesson 3 Bonding & the Periodic Table Diagram Quizlet
Aluminum (al) has 3 valence electrons. A) iodine b) aluminum c) carbon d) nitrogen Nitrogen (n) has 5 valence electrons. Carbon (c) has 4 valence electrons. Iodine (i) has 7 valence electrons.
PPT Chemical Bonding and Molecular Structure PowerPoint Presentation
Which element has the fewest valence electrons available for bonding? Carbon (c) has 4 valence electrons. The total number of electrons present in the outer. Aluminum (al) has 3 valence electrons. The element with the fewest valence electrons available for bonding is lithium (li), located in group 1, which has 1 valence.
[Solved] QUESTION 43 Which element has the fewest valence electrons? H
Iodine (i) has 7 valence electrons. Sodium (na) with atomic number 11 has the least number of valence electrons. The total number of electrons present in the outer. Nitrogen (n) has 5 valence electrons. This is typical for elements in group.
PPT Drawing Lewis Structures A Tutorial on Writing Lewis Dot
Which element has the fewest valence electrons available for bonding? Study with quizlet and memorize flashcards containing terms like how many valence electrons are available for bonding in bromine (br)?,. Carbon (c) has 4 valence electrons. Sodium (na) with atomic number 11 has the least number of valence electrons. This is typical for elements in group.
PPT Intro to Bonding Part 2 Covalent Compounds (Type 3 Binary
Aluminum (al) has 3 valence electrons. The element with the fewest valence electrons available for bonding is lithium (li), located in group 1, which has 1 valence. This is typical for elements in group. Sodium (na) with atomic number 11 has the least number of valence electrons. Thus, aluminum (al) has the fewest valence electrons, with only 3 available for.
Chemistry Shearer Standard 1 ppt download
Aluminum (al) has 3 valence electrons. The total number of electrons present in the outer. Which element has the fewest valence electrons available for bonding? Thus, aluminum (al) has the fewest valence electrons, with only 3 available for bonding. Iodine (i) has 7 valence electrons.
PPT Chapter 5 Atoms & Bonding PowerPoint Presentation, free
The total number of electrons present in the outer. Iodine (i) has 7 valence electrons. Which element has the fewest valence electrons available for bonding? Sodium (na) with atomic number 11 has the least number of valence electrons. Carbon (c) has 4 valence electrons.
Valence Electrons ELECTRONS AVAILABLE FOR BONDING CA Standards
Aluminum (al) has 3 valence electrons. Sodium (na) with atomic number 11 has the least number of valence electrons. A) iodine b) aluminum c) carbon d) nitrogen The total number of electrons present in the outer. Carbon (c) has 4 valence electrons.
A) Iodine B) Aluminum C) Carbon D) Nitrogen
Thus, aluminum (al) has the fewest valence electrons, with only 3 available for bonding. Study with quizlet and memorize flashcards containing terms like how many valence electrons are available for bonding in bromine (br)?,. Iodine (i) has 7 valence electrons. This is typical for elements in group.
Sodium (Na) With Atomic Number 11 Has The Least Number Of Valence Electrons.
Carbon (c) has 4 valence electrons. Which element has the fewest valence electrons available for bonding? Aluminum (al) has 3 valence electrons. The total number of electrons present in the outer.
Nitrogen (N) Has 5 Valence Electrons.
The element with the fewest valence electrons available for bonding is lithium (li), located in group 1, which has 1 valence.